Journée Thématique SFA – 2022

Gordon Research Conference – 2022
14 novembre 2022
22nd International Vascular Biology Meeting (2022)
14 novembre 2022Prix de la communication orale

Ilaria Del Gaudio – Post doctorant
Team PL Tharaux and E CAMERER
Kidney and Vascular Signalling: from Development to Disease
Paris-Cardiovascular Research Center PARCC
Paris
An important focus of my work within team 8 at the Paris Cardiovascular Research Center (PARCC) is to elucidate roles of the bioactive lipid mediator sphingosine 1-phosphate (S1P) in regulating vascular homeostasis. S1P signaling is of particular importance in resistance arteries, where it regulates flow-mediated vasodilation upstream of endothelial nitric oxide synthase. Several studies have indeed demonstrated that S1P can promote resistant artery tone in vitro, and plasma S1P correlates positively with systolic blood pressure in human hypertension. Yet the importance of S1P signaling in the context of redundant pathways and its mechanisms of engagement in vivo remain elusive, and it is unclear if it plays a causal role in the development of hypertension. S1P has also been shown to regulate cardiac function which could also impact blood pressure.
Thus, my project aims at investigating the mechanistic origin underlying S1P-mediated regulation of blood pressure and cardiac function.

Prix du poster

Marie Karam – PhD student
Team I.Brunet
Collège de France - Centre Interdisciplinaire de Recherche en Biologie (CIRB)
UMR7241/INSERM U1050
Paris
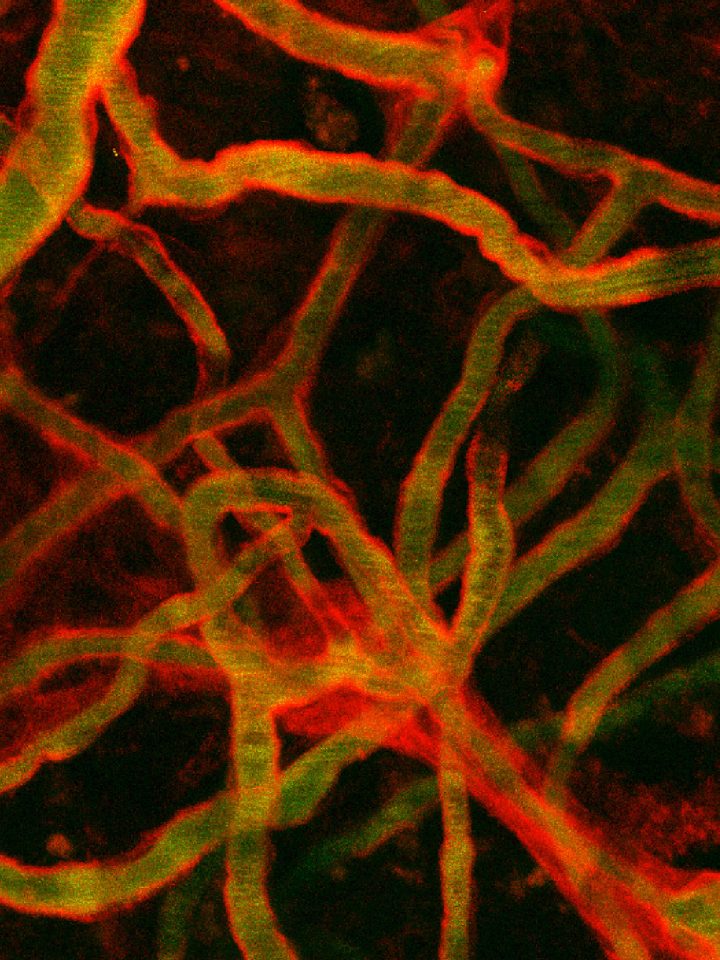
I started my phd in 2019, in the team of Isabelle BRUNET at the Collège de France. My thesis work mainly focuses on the role of perivascular macrophages (PVMs) in physiological and pathological conditions. The PVMs field is a booming field that is recently catching the eyes of a lot of scientists. Indeed PVMs implication in various aspects of neuronal homeostasis and function are growing, yet a lot of informations are still lacking and waiting to be uncovered. Using state of the art technique such as iDisco protocol, light sheet microscopy and traditional immunostaining technique, I have managed to characterize for the first time the PVMs vascular environment, coverage patterns, possible interaction with other blood brain barrier components, molecular heterogeneity and plasticity in Alzheimer’s disease.
Our work shows that PVMs are heterogeneous and can express not only CD206 but also the major lymphatic marker LYVE-1 at different ratios, highlighting the existence of subpopulations. LYVE-1+ PVMs (The lymphatic enriched population) have a tropism for veins. They can cover veins in a dense circumferential manner and interlace with the venous smooth muscle cells differently than the arterial ones. In addition, PVMs were shown to be plastic in an Alzheimer’s disease mice model (APP/PS1). They exhibited a loss of their lymphatic marker LYVE-1 in APP/PS1 compared to WT.
Our results suggest that LYVE-1+ PVMs may play a key role in brain clearance, and could be possibly used in the future to develop treatments in brain-drainage-defect diseases.

Nantes 10th Nov 2022 – Oral communication + Poster award